MedBridge Partners with Redox to Expand Its Network of EMR Integrations
HIT Consultant | March 03, 2020
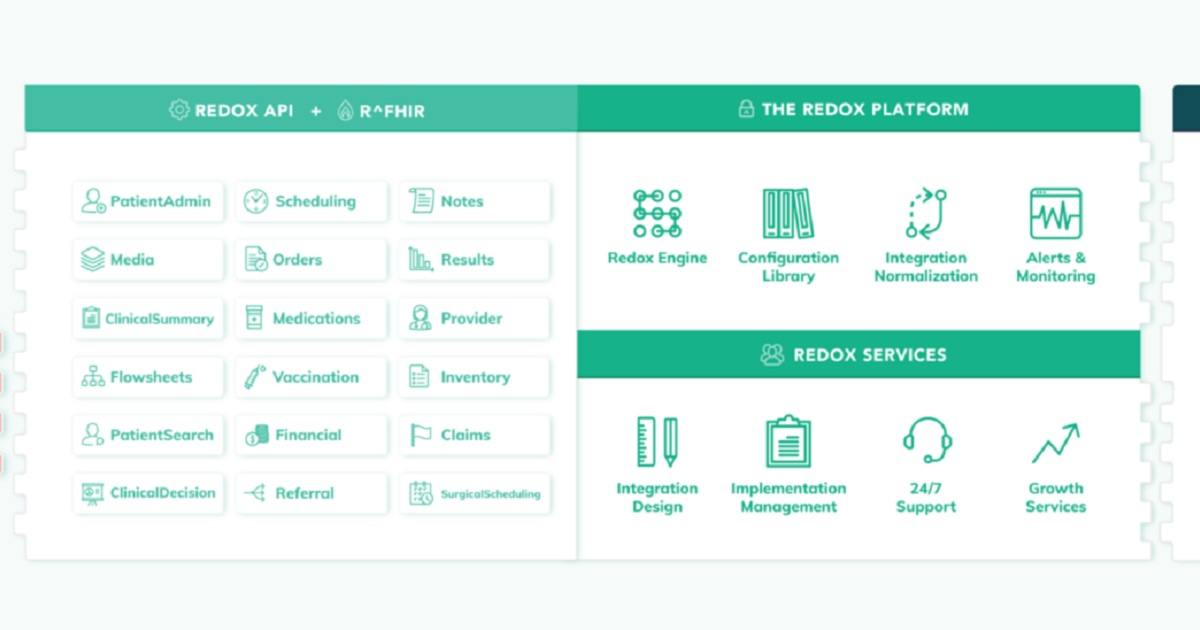
MedBridge, the industry-leading continuing education and patient engagement solution that enables healthcare organizations to improve outcomes and optimize care delivery announced today its partnership with Redox, a healthcare integration platform that allows software vendors to seamlessly integrate with healthcare organizations to securely exchange healthcare data. As part of the partnership, MedBridge will expand its current network of integrations to include leading EMRs, enabling healthcare professionals to quickly collect and record accurate patient information, saving time, and standardizing care coordination.