Bio Products Laboratory Announces Launch of ALBUMINEX® 5% and ALBUMINEX® 25% With Supply Immediately Available in the United States
Bio Products Laboratory | May 29, 2020
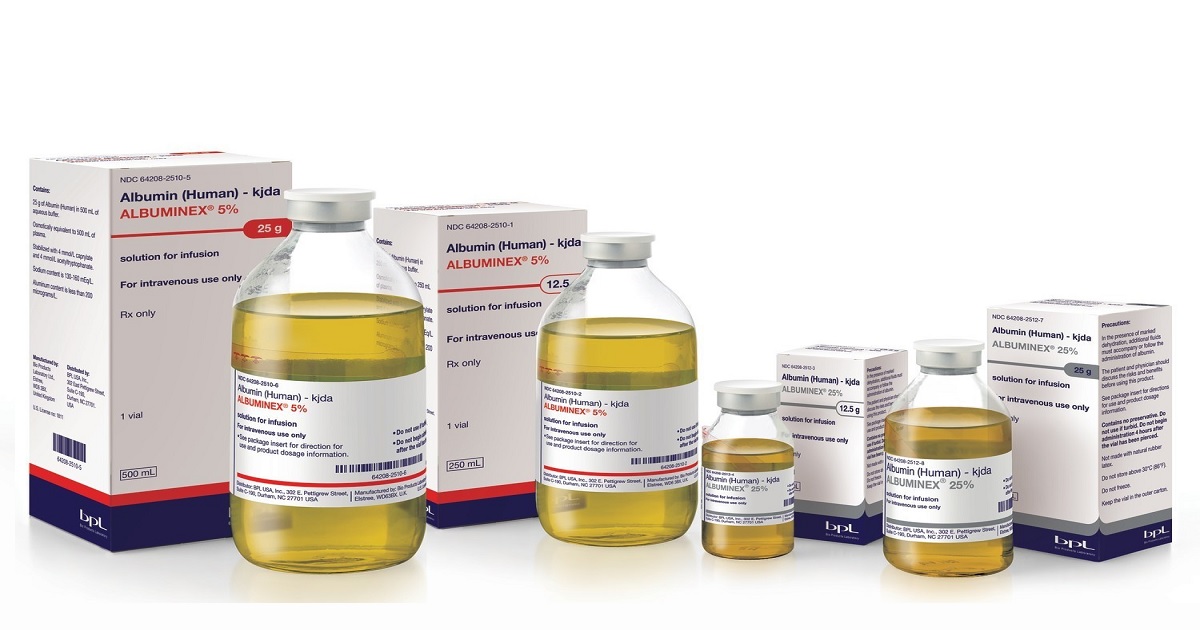
Bio Products Laboratory (BPL), a leading manufacturer of plasma-derived protein therapies with US headquarters in Durham, North Carolina, and worldwide headquarters and manufacturing facilities in Elstree, UK, today announced the US launch of ALBUMINEX 5% (human albumin) solution for injection and ALBUMINEX 25% (human albumin) solution for injection, with supply immediately available. ALBUMINEX 5% and ALBUMINEX 25% are approved by the US Food and Drug Administration for the treatment of hypovolemia, ascites, hypoalbuminemia including from burns, acute nephrosis, acute respiratory distress syndrome (ARDS), and cardiopulmonary bypass.